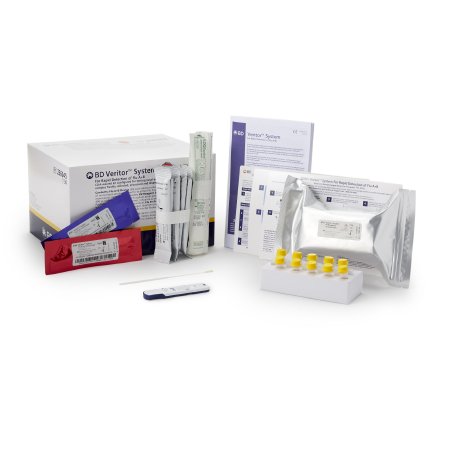
Respiratory Test Kit BD Veritor™ System Influenza A + B 30 Tests CLIA Waived
$419.39
:In stock
Share:
| SKU # | 785953 |
| Manufacturer # | 256045 |
| Brand | BD Veritor™ System |
| Manufacturer | BD |
| Country of Origin | United States |
| Application | Respiratory Test Kit |
| CLIA Classification | CLIA Waived |
| CLIA Classified | CLIA Waived |
| Contents 1 | Test Devices, Reactive Strips, Prefilled Reagent Tubes, Flocked Swabs, (2) Control Swabs |
| For Use With | For BD Veritor™ Plus Analyzer with BD Veritor™ InfoScan Module / BD Veritor™ Plus Analyzer / BD Veritor™ System |
| Is_Active_Vendor | Y |
| Is_DSCSA | N |
| Is_Discontinued | N |
| Is_Medical_Device | N |
| Lot_Tracking_Flag | Y |
| Number of Tests | 30 Tests |
| On_Allocation | N |
| Product Dating | McKesson Acceptable Dating: we will ship >= 60 days |
| Purchase Program Type | Standard Purchase |
| Reading Type | Machine Read |
| Sample Type | Nasal Swab / Nasopharyngeal Swab Sample |
| Specialty | Immunoassay |
| Supplier_ID | 488246 |
| Test Format | Test Device Format |
| Test Kit Type | Rapid |
| Test Method | Chromatographic Immunoassay |
| Test Name | Influenza A + B |
| Test Type | Infectious Disease Immunoassay |
| Time to Results | 10 Minute Results |
| UNSPSC Code | 41116144 |
Features
- Requires BD Veritor Plus Analyzer (not included) - see Item 1029527
- Includes 30 individually foil pouched test devices with one reactive strip
- Includes 30 tubes with 400 µL RV Reagent D
- Includes 30 flexible minitip flocked swabs
- Includes one each Control A+/B- Swab and Control B+/A- Swab
- Kits may be stored at 2 - 30°C: Do not freeze
- Reagents and devices must be at room temperature (15 - 25°C) when used for testing
- Acceptable specimens for testing with the BD Veritor System Flu A+B test include nasal swabs and nasopharyngeal (NP) swabs
- CLIA Waived









